Taisho Pharmaceutical Introduce Locoa(Esflurbiprofen), the Newest Treatment for the Management of Osteorthritis. | Hi everyone, transdermal patch is one of the famous way to treat pain like migraine and muscle pain. It is more safe and comfort method compare to taking medication.
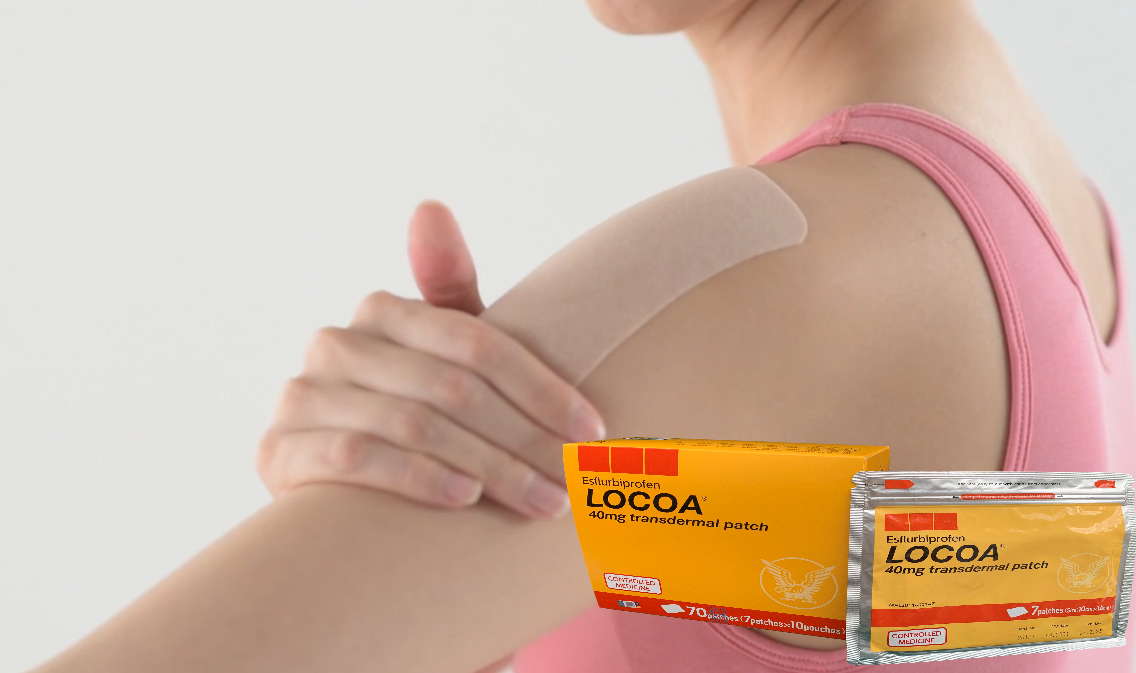
Recently, Taisho Pharmaceutical has launched LOCOA® (Esflurbiprofen), a transdermal patch, created to help the osteoarthritis patients to manage their pain that may be troubling their movements and daily activities.
 |
| Locoa Transdermal patch, |
Osteoarthritis is one of the most common joint disease and it is recognised as among the highest contributor of global disabilities.
“Once someone’s being diagnosed with osteoarthritis, there’s potential for the pain to occur more frequently thus it is vital to take preventive measures and while there is no cure for this incapacitating illness, treatments have improved significantly in the recent years and there’s a clear benefit in starting treatment at an early stage”, said Chief Operating Officer of HOE Pharmaceuticals, a subsidiary of Taisho Pharmaceutical Group, Mr. Tomoyuki Katsuta.
LOCOA® has been approved since 2016 in Japan and it was developed with the intention of giving more option to osteoarthritis patients in managing their pain more effectively.
It occurs more frequently in males before the age of 45 and in females after 45 years of age. Most common symptoms of osteoarthritis patients include joint pain, stiffness, swelling, loss of muscle bulk, limb deformity and grating or crackling sound or sensation.
The goal of treatment in osteoarthritis is to reduce joint pain and inflammation while improving and maintaining joint function. Developed in Japan, LOCOA® is a tape type patch with COX inhibition that is used to treat inflammation and pain for osteoarthritis.
LOCOA® offers high transdermal absorption ratio. LOCOA® has shown to provide high analgesic improvement.
Besides resting, stretching, weight loss management, physical therapy, occupational therapy or mechanical support devices, such as knee braces, osteoarthritis patients have an option with LOCOA® to manage their pain.
LOCOA® is proven to be safe on having a safety study based on a 52-weeks treatment and has minimal Adverse Drug Reactions (ADR). LOCOA® has a daily dosing of one patch only as the plasma concentration is well maintained throughout 24 hours.
 |
| Mr. Tomoyuki Katsuta |
Mr. Tomoyuki Katsuta also added, “We hope that patients who are on LOCOA® will be able to live their day-to-day life close to normal again without having to bear much pain”. He also highlighted that the launch of LOCOA® today in Kuala Lumpur is timely with the upcoming World Arthritis Day 2021 on October 12th, 2021.
It is worth noting that Taisho Pharmaceutical has decided to choose Malaysia as the first country outside Japan to introduce LOCOA®. According to the Department of Statistics Malaysia (DOSM), the number of people aged 65 years and above in Malaysia has increased steadily since the 1970s. Seeing the potential of Malaysia to be a country with high level of health consciousness, Taisho Pharmaceutical believes by introducing LOCOA® here, it would also benefit elderly Malaysian population.
The launch was officiated by Dr. Suhail Suresh, Consultant Orthopaedic from Sunway Medical Centre and witnessed by Mr. Tomoyuki Katsuta.
LOCOA® now can be found at all major pharmacies and healthcare facilities near you.
KKLIU 2358/2021
Stay safe and stay healthy gais!
About Taisho Pharmaceutical Co. Ltd
Taisho Pharmaceutical Co. Ltd is one of the leading pharmaceutical companies in Japan, has acquired HOE Pharmaceuticals in 2011. This acquisition strengthens the business in Malaysia and the growing Asian markets. The Company’s mission in its philosophy is to contribute to society by creating and offering top-quality pharmaceuticals and health-related products, as well as healthcare-related information and services in socially responsible ways that enrich people’s lives by improving health and beauty.
Being the foundation of all its corporate activities, Taisho recognizes that, more than ever, it is following in the tradition of SHINSHO (gentlemanship in business, in accordance with high social ethics), a phrase often quoted by the Company’s first honorary chairman, the late Shokichi Uehara. By keeping the SHINSHO business constantly in mind, and with an earnest desire to work for the consumers, it is Taisho’s intention to adhere strictly to its philosophy, and strives to contribute to all fields related to healthcare.
REFERENCES
National Institute of Aging (2017). Osteoarthritis. Retrieved from nia.nih.gov/health/osteoarthritis
National Health Service United Kingdom (2019) Overview: Osteoarthritis. Retrieved from https://www.nhs.uk/conditions/osteoarthritis/symptoms/
Yataba I, Otsuka N, Matsushita I, Kamezawa M, Yamada I, Sasaki S et al. Plasma pharmacokinetics and synovial concentrations of S-flurbiprofen plaster in humans. European Journal of Clinical Pharmacology. 2015;72(1):53-59.
Ikuko Yataba, Noboru Otsuka, Isao Matsushita, Hideo Matsumoto & Yuichi Hoshino (2017) Efficacy of S-flurbiprofen plaster in knee osteoarthritis treatment: Results from a phase III, randomized, active-controlled, adequate, and well-controlled trial, Modern Rheumatology, 27:1, 130-136, DOI: 10.1080/14397595.2016.1176624.
Ikuko Yataba, Noboru Otsuka et al, The Long-Term Safety of S-Flurbiprofen Plaster for Osteoarthrithis Patients: An Open-Label, 52-Week Study, Clin Drug Investig (2016) 36:673-682.
LOCOA® Approved Package Insert.
Department of Statistics Malaysia Newsletter (2017). Ageing. Retrieved from https://www.dosm.gov.my/v1/uploads/files/6_Newsletter/Ageing.pdf.






Thank you for dropping by. All comments are fully your responsibility.
Any comments are subjected to the Act 588 MCMC 1988. Comment wisely, and do it with open heart!
Happy Blogging.
For any inquiries, email : shamiera_osment@yahoo.com